NEW EUROPEAN REGULATION
Definition
“ Making available on the market” means any supply of PPE for distribution or use on the Union market in the course of a commercial activity, whether in return for payment or free of charge.
” Placing on the market” means the first making available of a PPE on the market of the union.
The European Commission
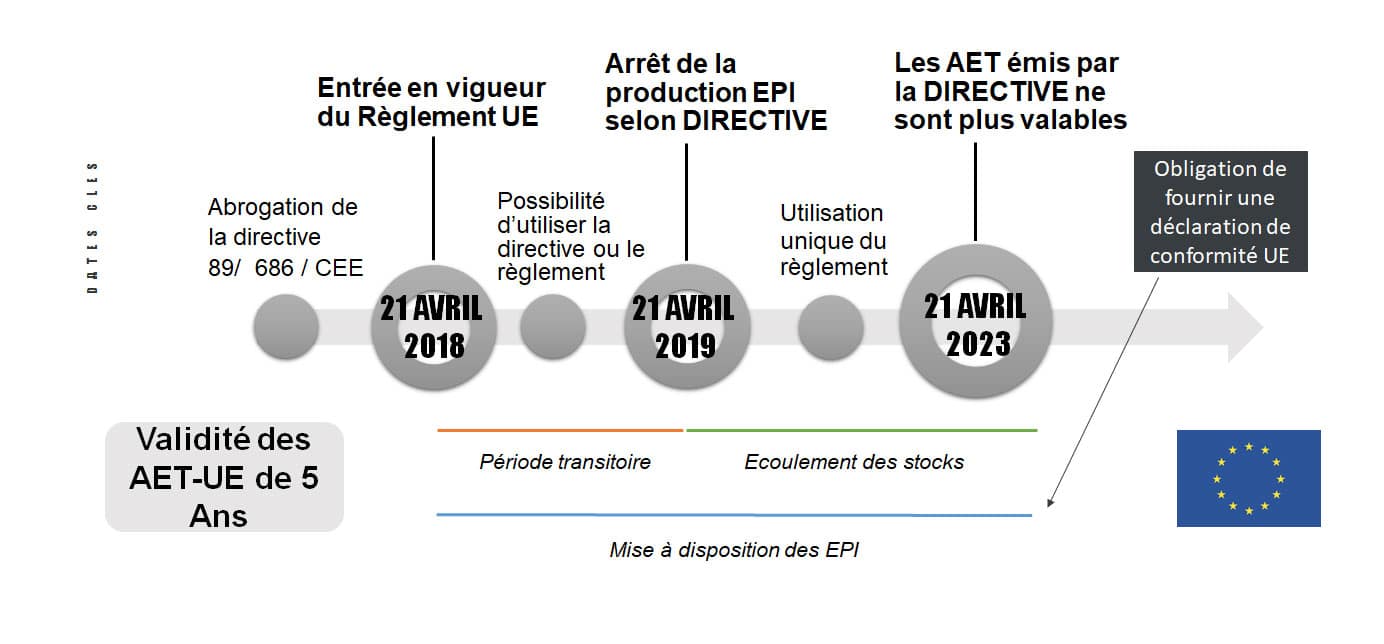
All Personal Protective Equipmentmanufactured after on April 21, 2019 must be compliant to EU Regulation 2016/425 even those with a valid EC Type Examination Certificate according to Directive 89/686/EEC.
The transitional period provided for by the regulation will generate market disruption, with manufacturers having to re-certify their entire ranges and notified bodies probably unable to meet the influx of applications.
It is possible to place on the market products that comply with the directive, for which there is a valid certificate (AET-CE), until 21 04 2023.
Therefore any PPE,
in the sense of an individual product,
that complies with the directive can no longer be placed on the market as of 21 April 2019, even if it has an EC-TEA.
For manufacturers, this means that the production of PPE in accordance with the directive will no longer be possible after 21/04/2019, or even earlier, since the time required to place the product on the market (transport of goods, customs, etc.) must be taken into account. …. Declarations of conformity referring to an EC-TEA shall be made in accordance with the regulations.
EU Statement
Declarations of conformity must include the lot number. In the sense of an individual product.
Attachment of a production batch to the EU declaration.
As of April 21, 2019, only PPE that complies with the regulation may be
placed on the market
. EC Type Examination Certificates (EU-TEA) and approval decisions can only refer to the regulation. An EU Declaration of Conformity referring to the regulation shall be established.
The
making available
of products complying with the directive is possible as long as the EC-TEA and, if applicable, the approval decisions for the control of PPE manufactured as indicated in Article 11 of the directive are valid, and at the latest until April 21, 2023.
Obligations of economic operators
“Manufacturer” any individual or legal entity that designs or manufactures PPE, or has it designed or manufactured and markets it under its own name or brand. It is subject to essential health and safety requirements and the establishment of technical documentation. This means the establishment of the EU declaration of conformity, the control procedures, the traceability as well as the instructions for use. It must cooperate with the national authorities.
“Importer” any natural or legal person established in the EU who places PPE from a third country on the EU market. It ensures that the conformity assessment procedure has been applied by the manufacturer. That the products are accompanied by an instruction manual. It keeps the declaration of conformity and the technical documentation available. It must cooperate with the national authorities. It ensures the conditions of storage and transport.
“Distributor” any individual or part of the supply chain, other than the manufacturer or importer, who makes PPE available on the market. He must check the marking, the presence of the declaration of conformity and the instructions for use. It ensures the conditions of storage and transport.
A importer or a distributor is considered as
a manufacturer
and is subject to the manufacturer’s obligations when he places PPE on the market under his own name or trademark or modifies PPE already placed on the market in such a way that compliance with the applicable essential health and safety requirements may be affected.
Conclusion
At first. It is imperative to streamline our entire range of protection.
Secondly, anticipate the realization of the tests if necessary according to the validity period of the test reports and the change of the standard or the claimed standards.
Thirdly, to update the technical files, the instructions for use and the EU declarations of conformity.
All this in order to be ready to have our TEA-EU as soon as possible.